Virus detection & Air sampling
Sampling virus-laden aerosols is a notoriously difficult endeavor. Here, our top scientists have shared best practices to use the Coriolis air samplers for virus detection in various types of environments.
The pandemic of Covid-19 has shown the vulnerability of our healthcare systems when faced with viral infections without a known treatment. Understanding the transmission behavior of airborne viruses is crucial to design the appropriate prevention and control measures to manage pandemics. A pre-requisite is the development of reliable environmental monitoring solutions to control for the presence of viruses in the air. Bertin Technologies has developed air samplers that can be used for the detection and monitoring of airborne viruses in a wide range of environments, from hospitals to office buildings, the Coriolis air samplers.
The detection of viruses in air samples presents many challenges: compared to other microorganisms, viruses are present in the air at a very diluted ratio which translates into the necessity of sampling a relatively large amount of air to have reliable analysis results – a few m3. The integrity of the virus also has to be maintained through each step of the workflow to have reliable virus viability estimates. Traditional air sampling devices are often impedimented by low airflow rates, which translates into a time-consuming sampling process for viruses.
On this page, our scientists have shared their tips for successful virus-laden aerosol sampling with the Coriolis air samplers.
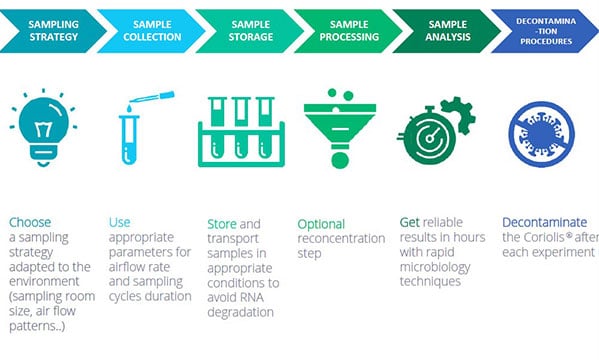
Best practices for virus detection in air samples with the Coriolis air samplers
1/ SAMPLING STRATEGY: choose a sampling strategy adapted to the environment (sampling room size, air flow patterns…)
There are many sampling strategies possible for the positioning of the Coriolis, depending on the room layout and the virus concentration in the air. For healthcare settings, the optimal positioning of the device is at an approximate distance of 1 meter from the patient bed and at approximately the same height as the patient’ head. If air samples are collected in several points of a patient’s room, it is preferable to start from the further distance away from the patient and then progress towards closer positions. For optimal particle collection, the device should always be placed on the trajectory of the airflow in the room.
When using the Long Time Monitoring option, it is preferable to inject sterile water (or a mix of sterile water and culture medium), rather than PBS, in order to avoid increasing the salt concentration in the cone.
2/ COLLECT AIR SAMPLES using appropriate parameters for airflow rate and sampling cycles duration.
- Airflow rate
It has been suggested that samplers with a flow rate higher than 200L/min may tend to degrade RNA virus during collection. For this reason, we recommend using an airflow rate of 200L/min or below. The Coriolis Compact can be used at 50 L/min. - Sampling duration
As viruses are most often present in the air at a very diluted concentration, most studies experimental design involves the collection of at least 1m^3 of air during each experiment, which corresponds to a sampling duration of a minimum of 5 min at a speed of 200L/min. When possible, a higher sampling duration should be preferred to collect the maximum volume of air possible, ideally 20 to 30 min at 200L/min. The Coriolis μ can collect air for up 1h on battery, and up to 6 hours with the Long Time Monitoring option on the main power. The Coriolis Compact can be used up to 8 hours on battery. - Sampling liquid
Surfactants such as Triton may affect the integrity of most viruses’ membranes. Therefore, we recommend against adding any surfactant to the collection liquid. The optimal collection liquid for virus-laden aerosols sampling is Phosphate Buffer Saline buffer. A culture medium such as DMEM or MEM is also a suitable alternative. On the other hand, most RNA shields should be avoided due to high evaporation speeds.
The recommended starting sampling volume liquid range is between 5 and 15mL.
When using the Long Time Monitoring option, it is preferable to inject sterile water (or a mix of sterile water and culture medium), rather than PBS, in order to avoid increasing the salt concentration in the cone.
With the Coriolis Compact, the collection is performed in a dry state. The biological particles can be recovered by adding the desired volume of buffer in the cone, which can allow for highly concentrated samples.
3/ SAMPLE STORAGE: store and transport samples in appropriate conditions to avoid RNA degradation
Before transportation, samples should be transferred from the cones into appropriate storage tubes. Samples can be stored for up to 24h at 4°C. For long-term storage they can be frozen in cryotubes at -20°C or -80°C.
4/ SAMPLE PROCESSING: optional reconcentration step
To obtain a final solution at a suitable concentration for analysis, most protocols require a reconcentration step with a tangential flow filtration device, such as the Amicon 100 kDa Amicon Ultra-15 (Millipore). However, in environments where the virus concentration step is sufficiently high, this step can be omitted by choosing a small starting volume of collection liquid (such as 5mL), then taking a small aliquot of around 150 μL for DNA or RNA extraction.
5/SAMPLE ANALYSIS with rapid microbiology techniques
As samples obtained with the Coriolis μ and the Coriolis Compact are in liquid form, they are compatible with all rapid microbiology techniques, such as qPCR, RT-qPCR, microarrays. Samples can also be analyzed with virus viability assays. It has been shown that liquid air samplers are less likely to damage viruses during sampling than dry air samplers [8], which implies that they are more adapted to virus viability studies.
6/DECONTAMINATION: decontaminate the Coriolis after each experiment
The Coriolis μ device should be decontaminated after each experiment. The cane, the air intake, and the sampling cones can be autoclaved. An alternative solution is to soak these parts in a commercial bleach solution. As most commercial bleach concentrations vary between 5.25 and 8.25%, a dilution of 1 part commercial bleach for 49 parts of water will make for a suitable disinfectant solution. Both the cane and the air intake may also be cleaned with ethanol 70% between each sampling. These solutions can be used to clean the external parts of Coriolis air sampler applied with a wet sponge or a rag.
The main unit of the Coriolis μ air sampler can be decontaminated using Hydrogen peroxide vapour (H202) and running the device in decontamination mode.
Alternatively, for virus sampling, the main unit can be decontaminated by running them with an ethanol-filled cone for 15min at 300L/min in a ventilated room. This will generate an aerosol of Ethanol that will disinfect the Coriolis main engine. Indeed, ethanol at 70% is a well-known disinfectant that has been shown to be effective on enveloped virus (hands or surfaces) even at lower concentrations. It is to be noted that, while this operation will destroy all viruses, spores won’t be eliminated.
All chemicals handling and decontamination procedures should also follow the user’s laboratory safety guidelines (regarding protective equipment…).